2024 ABN Fellowship
Investigating the consequences of HnRNPM mislocalisation in amyotrophic lateral sclerosis
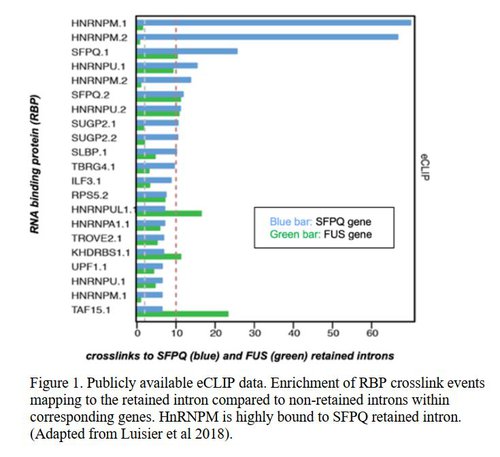
Amyotrophic lateral sclerosis (ALS) results in degeneration and death of motor nerve cells (motor neurons, ‘MNs’) which control walking, speaking, swallowing and breathing. It is fatal and untreatable. To develop treatments, we must understand precisely what causes MN degeneration. Important proteins which bind and regulate RNA messages are dysfunctional in MNs in ALS. I have discovered that another protein in this class, called HnRNPM, is abnormally ‘mislocalised’ from nucleus to cytoplasm in MNs in ALS. This is especially interesting since HnRNPM binds a specific abnormal RNA message in ALS and could be a new target for treatments.
To study ALS, we transform skin cells from ALS patients and healthy volunteers into stem cells then into MNs in the lab, and compare these. I hypothesise a loss of HnRNPM’s normal functions due to its mislocalisation in ALS. To model this, I will deplete HnRNPM in healthy MNs, then look for typical MN features of ALS, and for differences in RNA messages due to loss of HnRNPM's functions. I will also examine all interactions of HnRNPM with RNA messages in healthy and in ALS MNs. I will assess whether increasing HnRNPM levels can resolve features of ALS. This work will help us understand the contribution of HnRNPM to MN degeneration, and whether manipulating HnRNPM could treat ALS. If HnRNPM manipulation shows promise as an ALS treatment, development could progress to animal studies and subsequently clinical trials. From initial studies to approval of a treatment for patients could take approximately 10-15 years