
2021 Post-doctoral Clinical Fellowship
Combining plasma biomarkers with digital precision cognition metrics for Alzheimer’s Disease
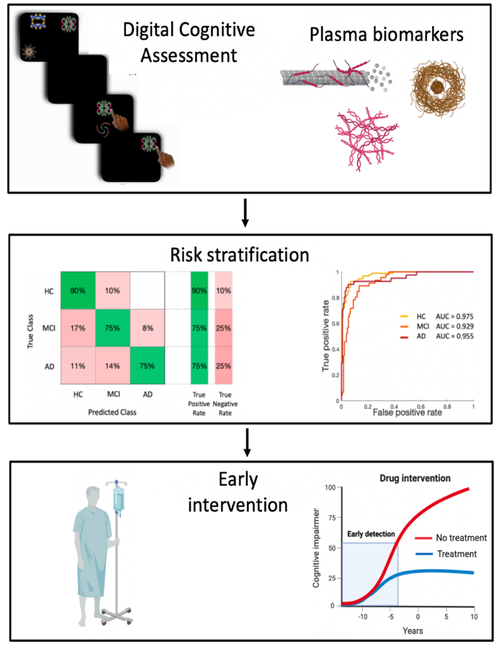
The advent of new disease-modifying treatments for Alzheimer’s Disease (AD) has led to an understanding that we need sensitive screening tests to stratify those who might benefit from early treatment and to track the effects of interventions. My aims are to test whether by combining plasma biomarkers with digital precision cognition metrics it is possible to develop more sensitive measures to detect AD early, stratify risk better and track progression over months.
This novel combination of digital cognitive assessment and plasma biomarkers will be measured longitudinally at 3 monthly intervals in a cohort of 60 healthy people, in patients at risk of developing AD, as patients with subjective cognitive impairment (SCI) and mild cognitive impairment (MCI), and AD patients.
Digital cognitive assessment provides more fine-grain measures of cognition than traditional neuropsychological tests, can be performed remotely and frequently, and therefore can capture preclinical signs of cognitive impairment earlier. In this project digital cognitive assessment will be performed though the Oxford Cognition online platform (https://oxfordcognition.org/).
Plasma biomarkers have recently emerged as a potential game-changer in AD. They are safe and easy to measure, cost-effective and both sensitive and specific. In this project I will be measuring plasma phospo-tau (p-tau181), Aβ oligomers (Aβ42 and 40), Neurofilament light chain (NfL) and Glial fibrillary acidic protein (GFAP), as they are useful to detect AD pathology early and track disease progression longitudinally, yielding comparable performances to cerebrospinal fluid (CSF) analysis.
I will then test the hypothesis whether the combination of digital cognitive assessment and plasma biomarkers can improve accuracy in patients’ stratification. We think this project will deepen our understanding of how a change in plasma biomarkers maps into a change in cognition even on a short timescale, which will be essential for better clinical endpoints in future trials.
Publications
Relationship of plasma biomarkers to digital cognitive tests in Alzheimer's disease
Diagnosis Assessment & Disease Monitoring, https://doi.org/10.1002/dad2.12590
Apr 2024