
2020 Post-doctoral Non-clinical Fellowship
Modulation and characterization of CXCL13-CXCR5+ CD8 T cell signalling as new translational opportunity to foster age-dependent repair and regeneration after nerve and spinal cord injuries
Aging has been linked to an increased prevalence of axonal injuries, which are characterized by inadequate regeneration and significant impairment [1-4]. However, the cellular and molecular understanding of the age-dependent regenerative decline is very sparse. Previous studies showed that an age-related impairment in de-differentiation and activation of Schwann cells (SCs) limits axonal regrowth in the injured peripheral nervous system (PNS), impairing sensory and motor recovery [5, 6]. In the central nervous system, following spinal cord injury, deletion of PTEN with an increase in mTOR signaling can only partially limit the aging-dependent axonal regenerative decay of corticospinal tracts [7]. These studies addressed some of the molecular mechanisms underpinning the aging-dependent molecular changes following an injury while aging in itself leads to profound modifications in cell signaling, metabolism, immunity, gene regulation, and protein translation in every tissue affecting homeostasis and predisposing to disease [8-12].
Recently, we discovered that the inflammatory cytokine lymphotoxin activates NFκB, which induces the neuronal expression of the chemoattractive protein C-X-C motif chemokine ligand 13 (CXCL13) that in turn recruits CXCR5+ CD8+ T cells in proximity of aged DRG neurons expressing MHC-I. CD8 T cells repress axonal regeneration of sensory DRG neurons by inhibiting the regenerative signals pAKT and pS6 via caspase 3 activation. Remarkably, CXCL13 neutralization with monoclonal antibodies prevents CXCR5+ CD8+ T cell recruitment to the DRG and reverses aging-dependent regenerative decline promoting neurological recovery following sciatic nerve injury (SNI). These data propose a novel aging-dependent mechanism restricting the axonal regenerative ability and offer a clinically suitable antibody-based manipulation of neuron-immune cell communication to promote repair.
This work was funded by the Guarantors of Brain post-doctoral fellowship and published in Science on 13th May 2022.
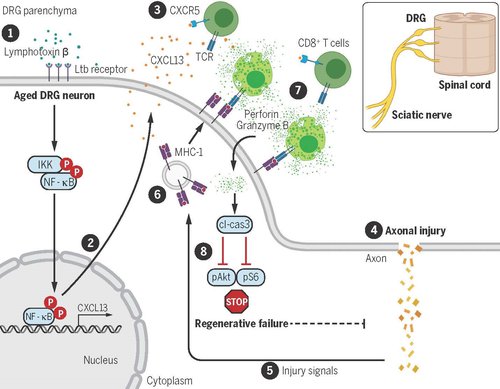
Figure 1. Aging-dependent regenerative failure in injured sensory neurons. (Zhou et al., Science, 2022)
References
Publications
Reversible CD8 T cell–neuron cross-talk causes aging-dependent neuronal regenerative decline
Science, Vol. 376, No. 6594
13 May 2022