
2022 ABN Fellowship
Characterising B Cell Tolerance in Autoimmune Encephalitis
Encephalitis is inflammation or swelling of the brain which leads to memory disturbance, mood changes and seizures. In the last decade, we have learned that this swelling can be caused by an immune system overactivity, which is as least as common as infectious causes. This is known as autoimmune encephalitis.
For reasons that we don’t yet fully understand, B cells, a key part of the immune system in establishing immune memory, recognise healthy brain tissue in error. These B cells then produce proteins called antibodies which target the brain to cause swelling and damage. The overactivity of B cells is likely to be caused by the failure of the normal checks and controls in the immune system, in a process known as immune tolerance.
Knowing where these breakdowns occur would allow us to:
I will analyse blood, lymph node and bone marrow samples from patients with autoimmune encephalitis and compare them to healthy and disease controls. I will examine B cells at each checkpoint in the immune to understand how they dysfunction and give rise to disease.
Once we understand where the immune system goes wrong, we can focus treatments that already exist on these targets. By exploiting this available knowledge, we can potentially rapidly bring treatments to patients, perhaps even within 2-3 years. In addition, the discovery of new targets in the faulty immune system will also allow us to develop new treatments.
As these checks and controls operate similarly across several conditions of the immune system, our findings will have wide ranging implications, across many neurological and non-neurological diseases.
Representative picture
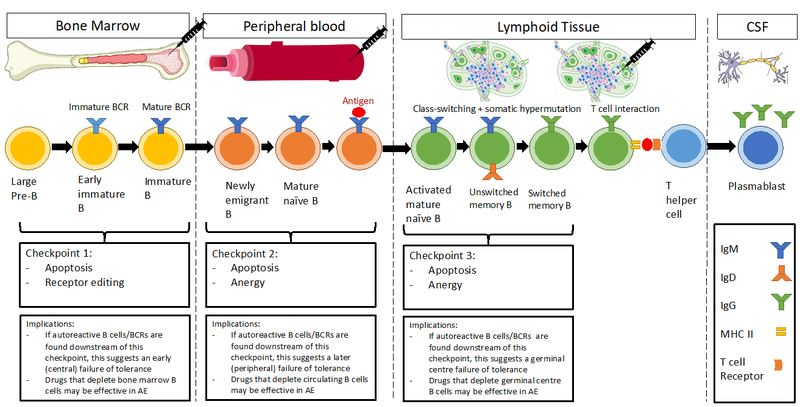
Figure 1. B cells pass through a series of checkpoints during development and maturation. Checkpoint 1 occurs in the bone marrow before B cells emigrate into peripheral circulation. Checkpoint 2 occurs in the peripheral blood as newly emigrant B cells mature into mature naïve cells. Upon exposure to cognate antigens, they may migrate to the germinal centres of lymphoid organs. Checkpoint 3 occurs as activated mature naïve B cells differentiate into unswitched memory B cells. Checkpoint 4 occurs as memory B cells undergo class switching and somatic hypermutation. At this point they acquire IgG and mature into plasmablasts, able to traffic into the CNS and secrete pathogenic autoantibodies. The novel use of lymph node aspirates allows the potential to study previously inaccessible B cell subsets in lymphoid organs, providing unique insights into germinal centre reactions in autoimmune disease.