
2021 Post-doctoral Non-clinical Fellowship
The role of hnRNP K in the pathogenesis of frontotemporal lobar degeneration
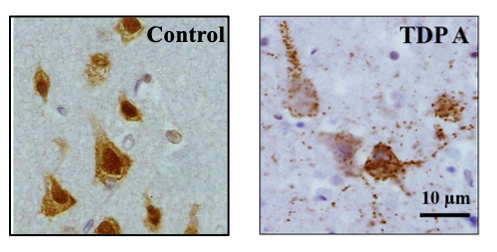
Frontotemporal lobar degeneration (FTLD) is a term used to describe a group of disorders characterised by the degeneration of the frontal and temporal lobes of the brain. There are currently five recognised subgroups of FTLD, three of which are characterised by the abnormal accumulation of a protein found in neurons: tau, transactive response DNA-binding protein (TDP-43) and fused in sarcoma (FUS). TDP-43 and FUS belong to the family of proteins known as heterogeneous nuclear ribonucleoproteins (hnRNPs), which bind to RNA within the nucleus of the cell and regulate its splicing. Abnormal splicing of RNA may lead to the introduction of genetic material which would not normally form part of the final RNA transcript, via the inclusion of cryptic exons. Presence of these cryptic exons can destabilise the RNA transcripts or lead to the production of toxic products. HnRNPs are mostly present in the nucleus of the cell, but in disease are known to move into the cytoplasm. Loss of TDP-43 from the nucleus in FTLD has been shown to lead to the inclusion of multiple cryptic exons and destabilised transcripts. HnRNP K, an hnRNP that shares structural and functional similarities with TDP-43, has been shown to be expressed in multiple brain regions in both normal and disease conditions. We have identified hnRNP K as being mislocalised from the nucleus to the cytoplasm in FTLD. Preliminary data suggests that the loss of hnRNP K from the nucleus can also lead to the inclusion of numerous cryptic exons. Due to the structural and functional similarities between hnRNP K and TDP-43, I will investigate the presence of cryptic exons in post-mortem human tissue and their relevance to disease. This project will extend the current knowledge on RNA processing in FTLD, which is an important step in the initial stages of neurodegeneration. Importantly, the work has the potential of identifying novel therapeutic targets for further investigations.
Publications
TDP-43 loss and ALS-risk SNPs drive mis-splicing and depletion of UNC13A
Nature 603:131–137. doi: 10.1038/s41586-022-04436-3
2022
HnRNP K mislocalisation in neurons of the dentate nucleus is a novel neuropathological feature of neurodegenerative disease and ageing
Neuropathol Appl Neurobiol 48(4):e12793. doi: 10.1111/nan.12793
2022
HnRNP K mislocalisation is a novel protein pathology of frontotemporal lobar degeneration and ageing and leads to cryptic splicing
Acta Neuropathol 142:609–627. doi: 10.1007/s00401-021-02340-0
2021
The role of hnRNPs in frontotemporal dementia and amyotrophic lateral sclerosis
Acta Neuropathol 140:599–623. doi: 10.1007/s00401-020-02203-0
2020